The study is divided into 3 phases.
Phase 1: Qualitative work and intervention development
We have spent around 160 hours observing what happens when people attend at the Liverpool Dental Hospital and NHS dental practices in Merseyside when they have a dental problem. There, we have spoken to 97 people, some of them more than once, who had a dental problem, sometimes for a few days and sometimes for weeks. We wanted to get a good idea about the reasons why people put off going to the dentist, and what might help them. We also asked them to help us understand what they think could help others, like their friends or family.
This has helped us design the intervention materials, which talk about the reasons why people put off going to the dentist, and what others have found helped them in getting back to a dentist. We have then shown the materials to lots of local people who have helped us to shape it into something which might be useful.
Phase 2: Feasibility study
We set up this study aiming to recruit 60 people into a feasibility trial run in 3 centres in Merseyside, over 3- month period. The first centre (a dental practice) opened for recruitment in January 2020 and fully recruited 20 people into the trial. The other 2 recruitment centres opened later, but unfortunately due to the COVID-19 pandemic, recruitment stopped in March 2020. We did however recruit 28 people to the study in a relatively short time window and so have been found to have met the objectives of the feasibility study, sufficient to progress to Phase 3 (the randomised controlled trial)
In the feasibility study patients were approached and asked whether they wanted to take part in the RETURN study when the attended for urgent dental care and were given information about the study. Half of the people taking part in the feasibility study received the intervention material and half just had their care as usual. People were randomly allocated to receive the intervention material or not.
We followed up people involved in the study 4 months after their visit by contacting them using the phone, e mail or post. We collected information such as whether they had visited a dentist for routine care since their urgent care appointment (the baseline visit).
During Phase 2 we observed the intervention being delivered and spoke to patients about what they thought about the intervention materials. This allowed us to do some refinement to the intervention materials.
We also incorporated some changes from feedback from a range of community and public engagement activities as well as from a stakeholder event involving the public, policy makers and dental teams. We will use the updated materials in Phase 3.
Phase 3: Randomised controlled trial
In Spring 2021 we are starting a randomised controlled trial in around 18 NHS dental practices throughout Merseyside and Cheshire which provide urgent dental care, and in Liverpool Dental Hospital. We will be including dental services which provide urgent dental care both within and outside normal working hours. We’lll be recruiting 1,180 patients in total and following them up to see whether they visited a dentist when they didn’t have a problem. Again, half of those patients (590) will receive the intervention materials.
Randomised controlled trial follow-up
We’ll stay in touch with everyone recruited to take part in the trial for 18 months to find out if they visit a dentist for a check-up and routine care during that time.
Everyone who takes part in the trial (both intervention and control groups) is shown this video which explains the follow-up involved in Phase 3.
During Phase 3, we will talk to around 50 people involved in the trial in more depth to explore in detail about their motivations and experiences of dental visiting during their time in the study throughout the 18-month period.
For Trial Participants
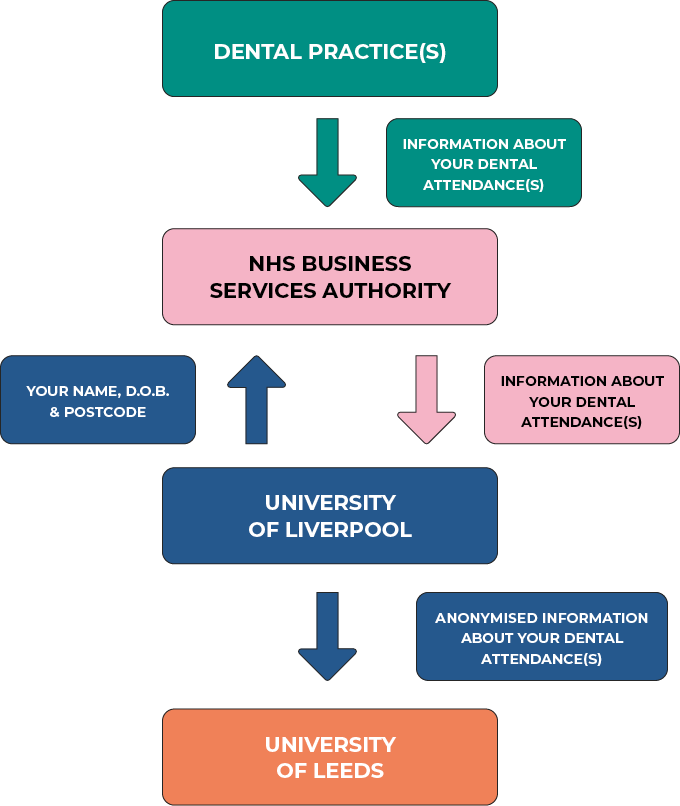
Further information about how we collect your data and which organisations see it
- What treatment you received
- Whether this was urgent or routine treatment
- When and which dental practice you attended
Withdrawal information for participants
Taking part is voluntary and you are free to withdraw at any time without giving a reason and your care, or legal rights, will not be affected. If at any point you decide to stop taking part, you will still get the usual information and treatment given by the dental team.
If you do stop taking part, we will use any information collected up until the time you stop taking part. However, the study team may need to continue to collect information about any side-effects you may have from taking part in this study.
Your rights to access, change or move your information are limited, as we need to manage your information in specific ways in order for the research to be reliable and accurate. If you withdraw from the study, we will keep the information about you that we already have. To safeguard your rights, we will use the minimum identifiable information possible.
Further qualitative work is also incorporated into Phase 2 and Phase 3 of the trial to refine the intervention after initial testing, and to build a detailed picture about any impact that the intervention might have, and how it works.




